How to Determine Which Gas Constant to Use
The ideal gas law equation The ideal gas law equation is pV nRT. P is the pressure of the gas measured in Pa.

Using The Ideal Gas Law To Calculate A Change In Volume Worked Example Video Khan Academy
We can plug this into the Ideal Gas Equation.
. We can write n number of moles as follows. The table given below comprised of the list of values of R in diverse units. The dimension of the gas constant is expressed in energy per unit mole per unit temperature.
Hence it is expressed in many units. N m M n m M. The value of the gas constant in SI unit is 8314 J mol 1 K 1.
And d is the nominal diameter of the pipeline in inches. Value Of Gas Constant. The ideal gas law formula states that pressure multiplied by volume is equal to moles times the universal gas constant times temperature.
V is the Volume in Metre cubes. P V n R T. Mg 2HCl MgCl2 H2.
However if we express R in units of L atmmol K its value is 008206. R gas constant. Use Daltons Law of Partial Pressures to determine the pressure of oxygen gas collected.
You will make an experimental determination of the number of moles of hydrogen molecules produced and the volume occupied by these molecules. Use the ideal gas law to determine the initial if needed and final volumes of the gas or any unknown. Be sure to keep your units straight.
It is the molar equivalent to the Boltzmann constant. The SI value of the gas constant is exactly 831446261815324 JK 1 mol 1. Its just a case of unit conversion.
Gas Constant In Different Units. If we are calculating using the ideal gas equation PVnRT then we use the 08206 because we will we will be calculating either L atm mol or K. A gas in a cylinder expands from a volume of 0110 m3 to 0320 m3.
R represents the ideal gas constant. Calculate the gas law constant R from your data using the ideal gas low. The letters are defined as follows.
The induvidual gas constant R for a gas can be calculated from the universal gas constant R u given in several units below and the gas molecular weight M gas. 069 is a constant factor for natural gas. Thus gas constant R value can be given as Gas constant R 8314459848 Jmol 1 K 1.
The value of R at atm that is at standard atmospheric pressure is R 83144598 Jmol-1K-1. P is the gas pressure measurement Pa. Also determine the initial and final.
The gas constant is inversely used in diverse disciplines. P Pressure atm mmHg Torr kPa The key is usually pressure. The digits inside the parentheses are the uncertainty in the measurement of gas constant value.
The ideal gas law states that PV nRT or in plain English that pressure times volume equals moles times the gas law constant R times temperature. V is the gas volume measurement m3. N is the substance amount measurement moles.
Calculate R using the van der Waals equation for O 2 a 1360 L2atm mol2 and b 3183 cm3 mol. The H 2 will be generated using this reaction. N is the amount of substance measured in moles.
Consider an ideal gas equation. P V nRT P V n R T. Mg s 2 HCl aq MgCl 2 aq H 2 g From the balanced equation you can see that there is a simple ratio between the number of molecules of Mg used and the amount of H 2 produced.
The universal gas constant is used in the equation PVNRT a relation of a perfect gas. Use a chemical reaction to generate and collect oxygen O2 gas over water. V is the volume of the gas measured in m³.
In SI units the real gas constant R is equal to 83145 Joulesmol K. Some say the symbol for the gas constant is named in honour of. T is the temperature of the gas measured in Kelvins.
The hydrogen gas is the product that is of interest to you in this experiment. The value of R can be expressed in multiple units. Where m is the mass of the gas and M is the molar mass.
The Gas Constant is the physical constant in the equation for the Ideal Gas Law. R The gas constant. The origin of the symbol R for the ideal gas constant is still obscure.
Use the ideal gas law PV nRT to calculate the Gas Constant R. The first approximation to all gases is a perfect gas which follows the relation that pressure volume nr atoms gas constant absolute temperature or PVNRT. Assuming O2 is an Ideal Gas Abstract.
T is the gas temperature measurement Kelvins. The properties of an ideal gas are all summarized in one formula of the form. The molar mass of an ideal gas can be determined using yet another derivation of the Ideal Gas Law.
PIRr 069 pd where PIR also written as r is the potential impact radius measured in feet. N no of moles of the gas. In this experiment you will determine the ideal gas constant using H 2 gas.
Determine the initial volume eqV_ 1 eq of the gas if provided. N number of moles. R R u M gas 1 In the imperial system the most common units for.
Usually the decimal is rounded to 8314. Now to evaluate the change in. The gas constant has the same unit as of entropy and molar heat.
If you need to find any of the aforementioned. The gas constant has the same unit as of entropy and molar heat capacity. The number of moles of hydrogen will be determined indirectly.
PV nRT Universal gas law Where P is the Pressure in Pascals. The R is also known as ideal gas constant or universal gas constant or molar constant. Ideal Gas Law Formula.
But the value of gas constant can be expressed using various units. P is the pressure in pipe or MAOP of the pipeline in psi. R is the ideal gas constant.
The value is independent of temperature. P V m MRT P V m M R T. The R is also known as ideal gas constant or universal gas constant or molar constant.
The Individual Gas Constant depends on the particular gas and is related to the molecular weight of the gas. T Temperature in Kelvin R fracNN_a. Henrys law shows that the concentration of a solute gas in a solution is directly proportional to the partial pressure of the gas over the solutionP KHC whereP is the partial pressure of the gas above the solutionKH is the Henrys law constant for the solutionC is the concentration of the dissolved gas in solutionC PKHC 24 atm2976 atm molLC 008.
Determination of Gas Constant R Learning Goals.

Ideal Gas Constant Definition Values And Units Chemistrygod

Chapter 2b Pure Substances Ideal Gas Updated 1 17 11

Chapter 2b Pure Substances Ideal Gas Updated 1 17 11
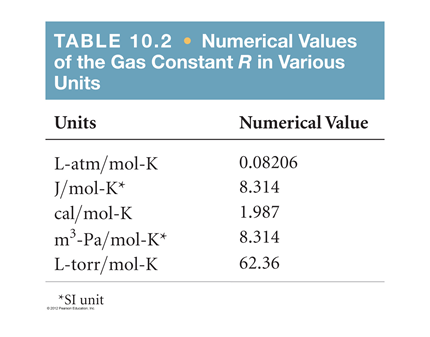
Solved Table 10 2 Numerical Values Of The Gas Constant R In Chegg Com
Specific Gas Constant Calculator
The Ideal Gas Law And Some Applications Introductory Chemistry

Gas Laws Equations And Formulas Youtube

Dalton S Law Of Partial Pressures The Bumbling Biochemist

Ideal Gas Law Practice Problems Youtube

Gas Constant Definition Formula Ideal Gas And Examples

Pv Nrt Use The Ideal Gas Law Youtube
What Value Of R Gas Constant Should Be Used Quora

Ideal Gas Law Calculator Pv Nrt

Dalton S Law Of Partial Pressures The Bumbling Biochemist

Using The Ideal Gas Law To Calculate Number Of Moles Worked Example Video Khan Academy
The Gas Laws A Boyle S Law Boyle S Law States If The Temperature Of A Gas Sample Is Kept Constant The Volume Of The Sample Will Vary Inversely As The Pressure Varies This Statement Means That If The Pressure Increases The Volume Will Decrease If The



Comments
Post a Comment